Temperature & Humidity: The Two Levers That Control Cel Life
An engineering-forward explainer of how temperature and moisture control the hydrolysis of cellulose acetate
How this fits into the Preservation Framework: This article represents the Acid Generation branch of the preservation model—the portion that explains why a cel generates acetic acid and how environmental conditions accelerate or slow that chemistry.
Humidity (aw / RH) and temperature together determine the hydrolysis rate constant k, which governs the pace of vinegar syndrome. Higher aw provides the water needed for chain scission, and elevated temperature increases the reaction rate exponentially.
No amount of ventilation, microchamber, or scavenging can stop this underlying reaction—those controls affect the emission side. The only way to suppress acid generation itself is through controlling T and aw, which is why they form the first pillar of the Preservation Framework.
TL;DR (targets you can actually use)
- Colder is exponentially better. Dropping from 70°F → 40°F can extend life by ~9× on temperature effect alone (Arrhenius; Ea ≈ 90 kJ/mol). Pair with drier air and totals can exceed ~25–40×.
- Control absolute humidity (AH), not just RH. At the same RH, cold air contains far less water than warm air. Cold storage often sits at low AH even when RH reads 40–50%.
- Mind the dew point. If a cel/enclosure is colder than the room’s dew point, condensation can form. This is why temperature and humidity must be considered together.
- Any drop in temp helps; even ~10°F colder can meaningfully extend life (Arrhenius).
- Stability beats perfection—avoid big daily swings in temp/RH.
- Understand AH vs RH: colder air holds less water at the same RH—this is why cool/cold storage wins.
- Moisture control (buffers/desiccants) and limiting acid buildup (ventilation/scavengers) further reduce risk.
1) Why temperature and humidity matter
Temperature drives reaction speed (hydrolysis)
Cellulose acetate slowly hydrolyzes (ester bonds cleave; acetic acid forms). Reaction rates follow the Arrhenius relationship, so colder storage slows the chemistry exponentially. A literature-class anchor is Ea ≈ 90 kJ/mol for CTA hydrolysis—high enough that small temperature changes have large effects.
Moisture enables the chemistry
Hydrolysis requires water. More water available → faster cleavage. Relative humidity (RH) is contextual to temperature; absolute humidity (AH) is the actual water mass per cubic meter and is the better cross-temperature control knob.
2) RH vs AH (and why AH is the control knob)
Comparing environments using ratios
RH (%) is the ratio of actual vapor pressure to saturation vapor pressure at that temperature. Because saturation rises steeply with T, the same RH at warmer temperatures contains more water than at colder temperatures.
AH (g/m³) is the actual mass of water per cubic meter—directly comparable across temperatures.
| Air Temp | 40% RH | 50% RH | 60% RH |
|---|---|---|---|
| 72°F (22.2°C) | 7.85 g/m³ | 9.82 g/m³ | 11.78 g/m³ |
| 50°F (10°C) | 3.76 g/m³ | 4.70 g/m³ | 5.64 g/m³ |
| 40°F (4.4°C) | 2.61 g/m³ | 3.27 g/m³ | 3.92 g/m³ |
Rule of thumb: at 40°F, 40–50% RH sits around ~2.6–3.3 g/m³ (dry in absolute terms).
3) Dew point (why temp + humidity interact)
Dew point is the temperature where the current air becomes saturated (RH = 100%). If a cel or enclosure is colder than the room’s dew point, condensation can form. Practically, this is the key reason temperature and humidity must be evaluated together rather than in isolation.
4) Arrhenius background: temperature and reaction rate
The Arrhenius equation links rate constant k to temperature T:
k = A · exp(–Ea / (R · T))
- A — pre-exponential factor (frequency of successful events)
- Ea — activation energy (energy barrier; ~90 kJ/mol for CTA hydrolysis)
- R — gas constant (8.314 J·mol⁻¹·K⁻¹)
- T — absolute temperature (Kelvin)
Lower temperatures reduce k exponentially, so relative life (≈ 1/k) rises sharply as storage gets colder. That’s the baseline mechanism behind the value of cool/cold storage—before even considering moisture.
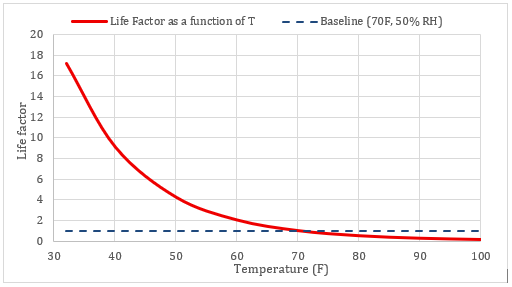
5) Modified Arrhenius: including moisture sensitivity
Temperature controls reaction speed, but water availability controls the fuel. Because RH is temperature-dependent, it can obscure how much water is actually present. Absolute humidity (AH) measures the real water mass (g/m³) available to drive hydrolysis.
Example: at 70°F/50% RH, AH ≈ ~9.2 g/m³. At 50°F/50% RH, AH ≈ ~4.7 g/m³—nearly half, even though RH is the same.
A moisture-aware model augments Arrhenius with an AH term:
k = A · AHn · exp(–Ea / (R · T))
For comparisons (target vs baseline):
Rate = ktarget / kbase = (AHt / AHb)n · exp( (Ea/R) · (1/Tt − 1/Tb) )
Relative Life = 1 / Rate
n is a water-sensitivity factor. In early, stable regimes moisture dependence may be weak (n small). As material approaches self-acceleration, n can rise—reflecting stronger sensitivity to available water.
Illustrative scenarios:
- Scenario 1: 70°F @ 50% RH → 50°F @ 50% RH (same RH, much lower AH)
- Scenario 2: 70°F @ 80% RH → 30°F @ 80% RH (huge thermal + AH drop)
- Scenario 3: 70°F @ 50% RH → 70°F @ 70% RH (thermal constant; moisture increases)
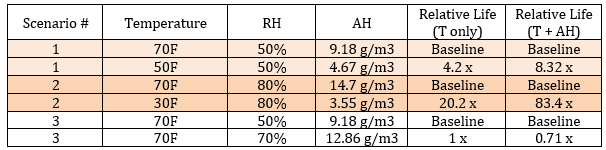
What this shows:
- At colder temperatures, AH can be dramatically lower at the same RH—further slowing hydrolysis beyond temperature alone.
- Colder almost always wins overall because temperature affects rate exponentially.
- Raising moisture at constant temperature (e.g., 70% RH vs 50% RH) increases rate even when the thermal multiplier is 1×.
6) Real-world storage & display environments (impact only)
Small differences in temperature and humidity can change decay rate by years or decades. Here’s how common environments impact hydrolysis and the two feedback loops—purely from an environmental standpoint.
6.1 Standard room display (typical home)
- Temperature: 68–75°F (20–24°C)
- Humidity: 40–60% RH (often fluctuating)
- Light: Moderate to high (room/daylight)
Impact: Fastest rate of decay. Hydrolysis and both loops remain active. Acid and moisture cycles build.
6.2 Controlled display room
- Temperature: 65–70°F (18–21°C), stable
- Humidity: 35–45% RH, controlled
- Light: Low-heat, UV-filtered, dim/indirect
Impact: Much safer than standard display; rate still non-trivial but reduced.
6.3 Cool storage
- Temperature: 50–60°F (10–15°C)
- Humidity: 30–40% RH
- Light: Dark/minimal
Impact: Hydrolysis slows dramatically; AH lower; lifespan multiplies vs room conditions.
6.4 Cold storage (archival)
- Temperature: 32–45°F (0–7°C)
- Humidity: 30–40% RH (controlled)
- Light: None
Impact: Hydrolysis nearly stops; both loops suppressed; acid production very slow.
6.5 Frozen storage (maximum preservation)
- Temperature: −4 to 14°F (−20 to −10°C)
- Humidity: Vapor effectively ~0% (water locked as ice)
- Light: None
Impact: Hydrolysis essentially halted; highest preservation level.
7) Key takeaways
- Cool slows it. Dry starves it. Together, they win. Temperature reduces rate exponentially; moisture supplies the fuel.
- Absolute humidity beats RH for cross-temperature reasoning. Cold air at 40–50% RH can still be very dry in absolute terms.
- Small improvements matter. Even modest drops in temperature and AH can add years or decades of life.
- Temperature and humidity interact. Dew point risk is the clearest proof that you must consider both together.
Want to compare environments quantitatively? See our calculator: Life Pro.