The Chemistry of Hydrolysis
Where Vinegar Syndrome begins
Cellulose acetate, the base material of most animation cels, undergoes progressive molecular breakdown over time. Through hydrolysis, acetyl ester bonds cleave, releasing acetic acid and regenerating hydroxyl ( acid-catalyzed autocatalysis (more acid → faster hydrolysis) and hydrophilicity amplification (more –OH ) groups on the cellulose backbone. This single reaction triggers two self-reinforcing feedback loops: –OH groups → higher moisture uptake → faster hydrolysis). The result is both molecular decay and visible physical symptoms—curling, embrittlement, shrinkage, and the unmistakable vinegar odor.1. Introduction
Hydrolysis is the fundamental chemical reaction driving the deterioration of cellulose acetate films. In this process, water cleaves ester bonds, releasing acetic acid and regenerating hydroxyl groups on the polymer backbone. This reaction is the core of vinegar syndrome—a self-catalyzing cycle of acid production and accelerating decay.
To fully understand why this happens, we must first examine how the material is built—from glucose, to cellulose, to cellulose acetate.
2. From Glucose to Cellulose Acetate
Cellulose acetate originates from a natural polymer, cellulose, which itself is made from repeating units of glucose. Understanding each step of this transformation explains both the useful properties of cellulose acetate and why it eventually degrades by hydrolysis.
2.1. Glucose
Glucose (C₆H₁₂O₆) is a six-carbon sugar whose ring structure contains several hydroxyl (2, 3, and 6 are the three reactive positions most involved in bonding and substitution reactions.–OH) groups. These hydroxyls enable two critical processes: linking glucose units into cellulose and later being replaced by acetyl groups to form cellulose acetate. In β-D-glucose, the hydroxyls on carbons
2.2. Formation of Cellulose
Cellulose is a linear polymer of glucose linked through β(1→4) glycosidic bonds. Each bond forms when the hydroxyl group on carbon 1 of one glucose unit reacts with the hydroxyl on carbon 4 of the next, releasing water during polymerization:n C₆H₁₂O₆ → (C₆H₁₀O₅)ₙ + n H₂OThis reaction produces the repeating anhydroglucose unit (C₆H₁₀O₅) — the structural backbone of cellulose. Extensive hydrogen bonding between adjacent chains gives cellulose its crystallinity, rigidity, and resistance to most solvents.
2.3. Acetylation of Cellulose
To transform cellulose into cellulose acetate, the hydroxyl groups on each glucose unit are replaced with acetyl (–acetylation. This is achieved by immersing cellulose in a mixture of acetic anhydride and acetic acid, with a small amount of sulfuric acid as catalyst. The reaction proceeds as follows:COCH₃) groups through a reaction known as
(C₆H₁₀O₅)ₙ + 3n (CH₃CO)₂O → (C₆H₇O₂)(OCOCH₃)₃ₙ + 3n CH₃COOHEach hydroxyl group (degree of substitution (DS)—the average number of acetyl groups per glucose unit (0–3)—determines the specific type of cellulose acetate produced.–OH) is converted into an ester linkage (–O–COCH₃), and acetic acid (CH₃COOH) is released as a byproduct. The
2.4. CTA and CDA
- Cellulose Triacetate (CTA) — DS ≈ 3.0. Nearly every reactive hydroxyl is acetylated, making the polymer highly hydrophobic, transparent, and dimensionally stable. Used in high-grade film bases and magnetic tape.
- Cellulose Diacetate (CDA) — DS ≈ 2.0. Fewer acetyl groups make it more flexible and soluble in acetone, but also more hydrophilic and less chemically stable. Common in molded parts and early motion picture films.
The level of acetylation directly influences the film’s processability, moisture response, and long-term stability. Acetylation reduces inter-chain hydrogen bonding, turning a rigid, fibrous polymer into a smooth, thermoplastic material that can be cast or molded—ideal for transparent films such as animation cels.
2.5. The Purpose of Each Phase
Once the desired level of acetylation is reached, the material is purified and cast into sheets or molded parts. This transformation—from glucose to cellulose acetate—defines the polymer’s chemistry and processability, but it also sets the stage for its eventual degradation. Over time, water reverses the esterification process through hydrolysis, regenerating both hydroxyl groups and acetic acid.
3. Transition to Hydrolysis & Degradation
With acetylation complete, the cellulose backbone now carries ester linkages (Achilles’ heel of the polymer. In the presence of water and trace acidity, they slowly cleave, regenerating hydroxyl (–O–COCH₃) that make the material transparent, thermoplastic, and initially more moisture-resistant than raw cellulose. However, these same ester bonds are also the –OH) groups and releasing acetic acid (CH₃COOH).
Each cleavage increases hydrophilicity and lowers local pH—two changes that accelerate further hydrolysis. This self-reinforcing cycle is the chemical foundation of vinegar syndrome.
The next section explores this reaction in detail—its kinetics, environmental drivers (temperature and humidity), and how molecular changes lead directly to the visible symptoms of decay.
4. Ester Bonds and Hydrolysis
An ester bond is the linkage that attaches an acetyl group to the cellulose backbone: the oxygen of cellulose (Cellweak point in the polymer.–O–) joins to the carbonyl of the acetyl group (–C(=O)CH₃). This bond makes cellulose acetate transparent, moldable, and initially more moisture-resistant. However, it is also the
- Cellulose triacetate (CTA): ~3 ester bonds per glucose unit
- Cellulose diacetate (CDA): ~2 ester bonds per glucose unit
- Cellulose monoacetate (CMA): ~1 ester bond per glucose unit
Each ester bond represents both:
- a stored acetic acid molecule (potential acidity), and
- a site that can revert back to a hydrophilic hydroxyl (–OH) if the bond is broken.
When hydrolysis occurs, water attacks the ester linkage:
Cell–O–C(=O)CH₃ + H₂O → Cell–OH + CH₃COOH- One ester bond cleaved → one acetic acid molecule released.
- One ester bond cleaved → one hydroxyl (–OH) restored.
(1) releases free acetic acid (vinegar smell),
(2) creates a new hydrophilic site (increasing water attraction).
Both changes accelerate further decay.
5. Acid Autocatalysis (Hydrolysis Loop)
Cellulose acetate does not decay at a constant rate. It decays slowly at first, then accelerates. The reason is that hydrolysis is an acid-catalyzed reaction: acetic acid both causes and results from ester bond cleavage.
- Hydrolysis releases acetic acid.
- The acid lowers local pH.
- Lower pH dramatically speeds up hydrolysis.
- Faster hydrolysis releases even more acid.
Figure 3. Acid-catalyzed feedback loop
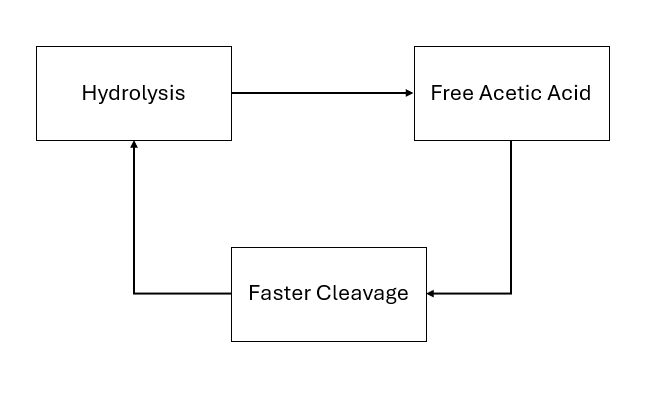
Hydrolysis releases acetic acid, lowering pH and accelerating further cleavage — a classic autocatalytic loop.
6. Hydrophilicity Amplification (Water-Driven Loop)
Each time an ester bond is cleaved, the cellulose backbone gains a new hydroxyl (–OH) group. Hydroxyls are highly hydrophilic, so the polymer begins to attract and hold more water from the environment. This creates a second feedback loop, independent of acidity.
- Hydrolysis restores –OH groups.
- More –OH groups increase hydrophilicity.
- Higher hydrophilicity raises moisture uptake (EMC).
- More absorbed water accelerates further hydrolysis.
Unlike the acid loop (which can be interrupted by neutralization or ventilation), the hydrophilicity loop is irreversible. Once hydroxyl groups replace acetyls, the polymer permanently becomes more polar, more moisture-sensitive, and more prone to dimensional change.
Together, the acid and hydrophilicity loops explain why degradation starts slowly but eventually becomes rapid and difficult to stop.
7. Chemical–Physical Linkages
The chemistry of hydrolysis directly drives the physical symptoms collectors observe:
- Increased hydroxyl density raises equilibrium moisture content (EMC), causing the film to absorb more water and expand dimensionally.
- Uneven moisture uptake across layers creates differential expansion, leading to curling, cockling, and warping.
- As polarity increases, plasticizers migrate out of the film, leaving the polymer backbone rigid and prone to embrittlement and cracking.
- Accumulated acetic acid lowers pH, producing vinegar odor and weakening paint adhesion.
- As degree of substitution (DS) drops and the polymer backbone loses acetyl protection, the film becomes opaque, brittle, and mechanically unstable.
8. Conclusion
Cellulose acetate degradation is fundamentally driven by hydrolysis. Each cleaved ester bond releases acetic acid and restores a hydrophilic hydroxyl group, triggering two reinforcing feedback loops:
- Acid autocatalysis — more acid lowers pH and accelerates hydrolysis
- Hydrophilicity amplification — more –OH groups increase moisture uptake and further fuel hydrolysis
The acid loop can be interrupted through ventilation, neutralization, or chemical resets. However, the hydrophilicity loop is irreversible—once acetyl groups are lost, the polymer permanently becomes more moisture-sensitive and dimensionally unstable.
For this reason, resets alone are not enough. Only the combination of resets (to remove acid) and controlled storage (to slow both loops) can meaningfully extend the lifespan of animation cels.
Key Terms & Definitions
- Hydrolysis
- Chemical cleavage of an ester bond by water, regenerating a hydroxyl (–OH) on cellulose and releasing acetic acid (CH₃COOH).
- Ester Bond
- The linkage
O–C(=O)–Rformed when a hydroxyl oxygen of cellulose bonds to an acyl group (e.g., acetyl). - Acetyl Group
- The acyl fragment
–C(=O)CH₃. When attached to cellulose via oxygen it appears as–O–C(=O)CH₃(often writtenOCOCH₃). - Degree of Substitution (DS)
- Average number of acetyl groups per glucose unit (0–3). Higher DS (CTA) = more acetyl protection; lower DS (CDA/CMA) = higher hydrophilicity and faster decay.
- Autocatalysis
- A self-accelerating reaction in which the product (acetic acid) catalyzes further hydrolysis by lowering pH, causing the reaction rate to increase over time.
- Hydrophilicity
- Affinity for water. As hydrolysis restores hydroxyl (–OH) groups, the polymer becomes more polar and absorbs more moisture, which in turn accelerates further hydrolysis.
- Cellulose Triacetate (CTA)
- Cellulose with ~3 acetyls per unit (DS≈3). Hydrophobic, dimensionally stable, used in film bases.
- Cellulose Diacetate (CDA)
- Cellulose with ~2 acetyls per unit (DS≈2). More flexible and soluble than CTA, used in molded parts/early films.